Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. An atom has no rigid spherical boundary, but it may be thought of as a tiny, dense positive nucleus surrounded by a diffuse negative cloud of electrons. The value of atomic radii. Unlike a ball, an atom doesn't have a fixed radius. The radius of an atom can only be found by measuring the distance between the nuclei of two touching atoms, and then halving that distance. As you can see from the diagrams, the same atom could be found to have a. An atomic radius can be described in two ways. Nonbonding atomic radius, or van der Waals radius of an atom, is one-half of the distance between adjacent nuclei in the atomic solid. Conversely, a bonding atomic radius, or covalent radius, distinguishes between metals and nonmetals.

More Videos For Atomic Radii »
Let's go ahead and talk about some periodic trends that we can gather from the periodic table. So let's start with atomic radii as that's a little bit simpler and then we'll move in to talking about ionic radii.
So, what do I mean when I say atomic radii? So basically within each group, remember groups or families are the columns on the periodic table. The atomic radius tends to increase from top to bottom, okay. So at the top you have a smaller atomic radius, as you go down the atomic radius gets larger. So why is that? That's because going down a column, the outer electrons are further from the nucleus. So the valence electrons. As you go down the columns, your principle quantum number increases, right? So then you have larger orbitals that are further away from the nucleus. So within each row though, or the period, the atomic radius tends to decrease from left to right.
So as you go from the left to the right, your atomic radius tends to go down, and this is due to something called the 'effective nuclear charge' or 'Z effective.' So what that means is that as you go to the right hand side of the periodic table, the increase in this nuclear effective nuclear charge, it draws the valence electrons in closer and so the atomic radius goes up. So as you go this is just a depiction again of the periodic table. Pretend this is it. So as you go down the columns, the atomic radius increases and as you go over to the right, the size of the atomic radius decreases.
So let's talk about ionic radii. So, similar to the atomic radii, this is the radii of ions and that's based on the distances between the ions and the ionic compounds. Alright so if I'm saying an ionic compound I'm saying you've at least two species. One of them is going to be positively charged or a cation, one of them will be negatively charged and an anion. So then the size depends on the number of electrons it possesses and where those electrons reside, so in which valence shell.
So you can have the formation of a cation, right? So the removal of an electron and that will create space because it reduces electron-electron repulsions within the ion. So cations tend to be smaller than their parent atoms. So sodium with no charge is going to be larger than sodium plus ion. So the opposite is true of anions right, because with an anion you're adding in a negative charge, so then anions will be larger than their parent atoms. So fluorine with no charge is going to be smaller than fluorine with a negative charge.
So ions with the same charge their size increases going down a column. So if we take a group one element, say we're comparing cesium plus and sodium plus, cesium is going to have a larger ionic radius than does sodium.
So one other concept that might come up when you're thinking about atomic and ionic radii, might be this definition of an isoelectronic series and so that's when a group of ions containing the same number of electrons are being compared. Okay, so here I've written oxygen two minus fluorine minus one, sodium plus one and magnesium plus two. These guys are part of an isoelectronic series because they all contain ten electrons. And that's atomic and ionic radii.

Atomic Radius Of K
Atomic radius of all the elements are mentioned in the chart below.
(Note: Below mentioned radii are the van der Waals radius in picometer (pm)).
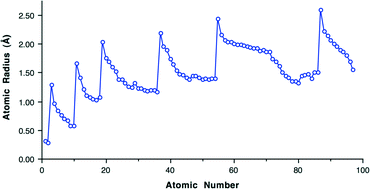
Images For Atomic Radii
Bonus Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
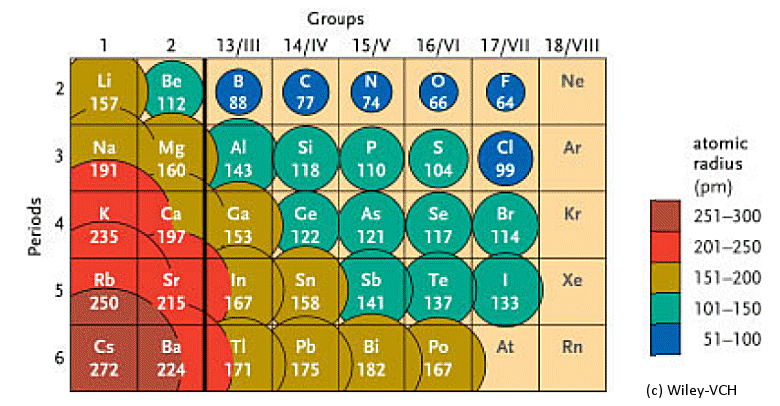
2). You will get the detailed information about the periodic table which will convert a newbie into pro.
3). You will also get the HD images of the Periodic table (for FREE).
Checkout Interactive Periodic table and download it’s high resolution image now (It’s FREE)
